
At KODA Ideaworks, we create practical medical innovations that solve urgent safety failures in healthcare. Our flagship product, HypoHolder, is FDA-registered Class I safety-engineered medical device, commercially available, and redefining hypodermic needle safety across surgical, clinical, and field settings. Designed to address the 800,000 needlestick injuries that occur annually, many during routine, two-handed handling, HypoHolder uses a patented, one-handed c platform to enable safe, controlled use in even the most high-pressure environments.
HypoHolder is more than a device, it’s a complete hypodermic needle safety solution, proven to reduce needlestick injuries, protect workflows, and meet the highest standards of care across surgical, clinical, and field settings.
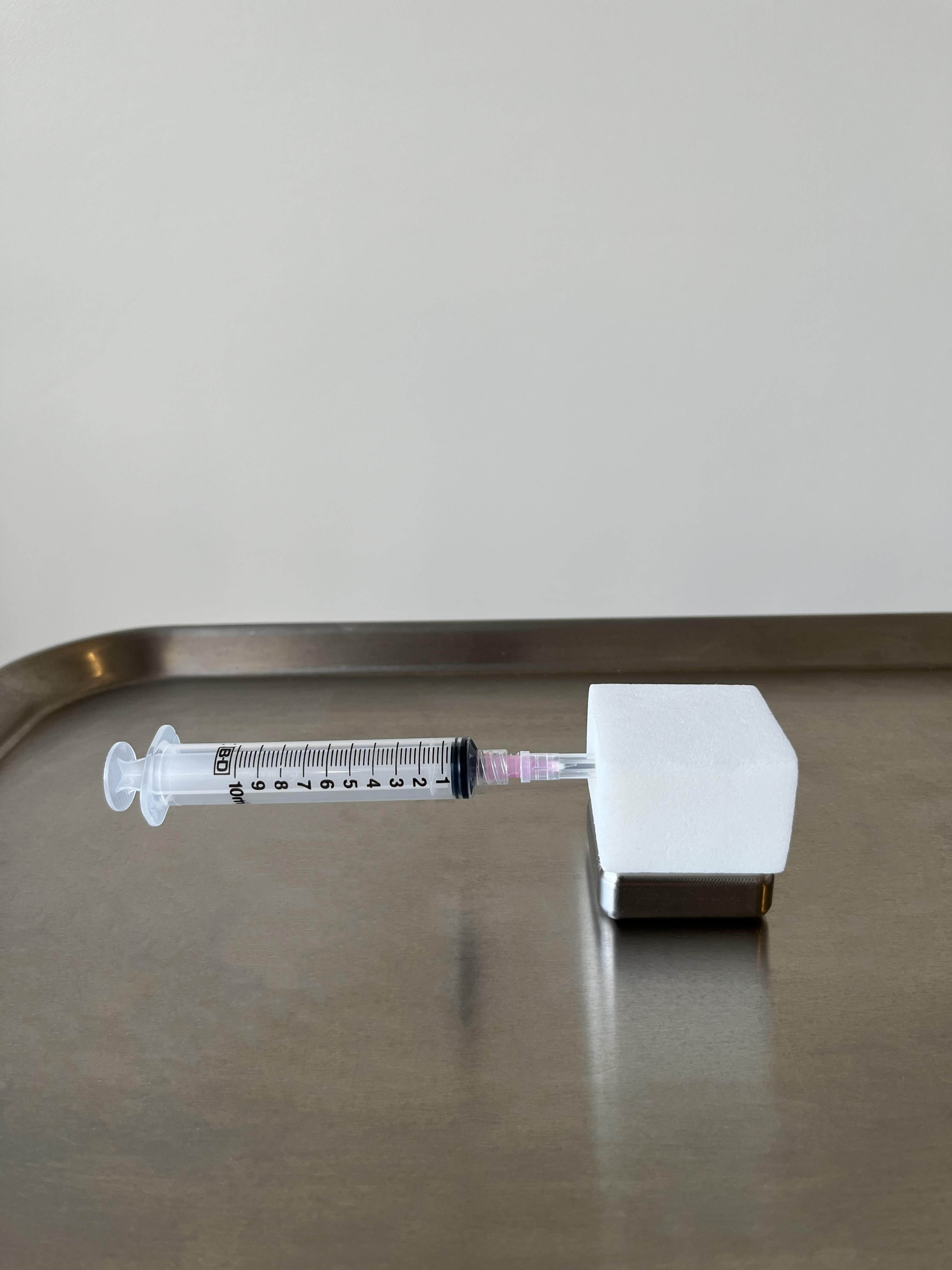
HypoHolder’s intuitive, one-handed operation enables safe hypodermic needle handling without needing to hold the device in place, with either magnetic stabilization (Surgical HypoHolder), weight and friction (Clinic HypoHolder) or temporary non-residue adhesive (Lite HypoHolder). Lightweight, durable, and compatible with a wide range of luer lock hypodermic needles, it’s designed for rapid adoption with minimal training. Beyond ease of use, HypoHolder supports institutional compliance with OSHA, CDC, VA, and Joint Commission standards, aligns with the Needlestick Safety and Prevention Act, and meets AORN’s 2025 Sharps Safety Guidelines for engineered injury prevention.